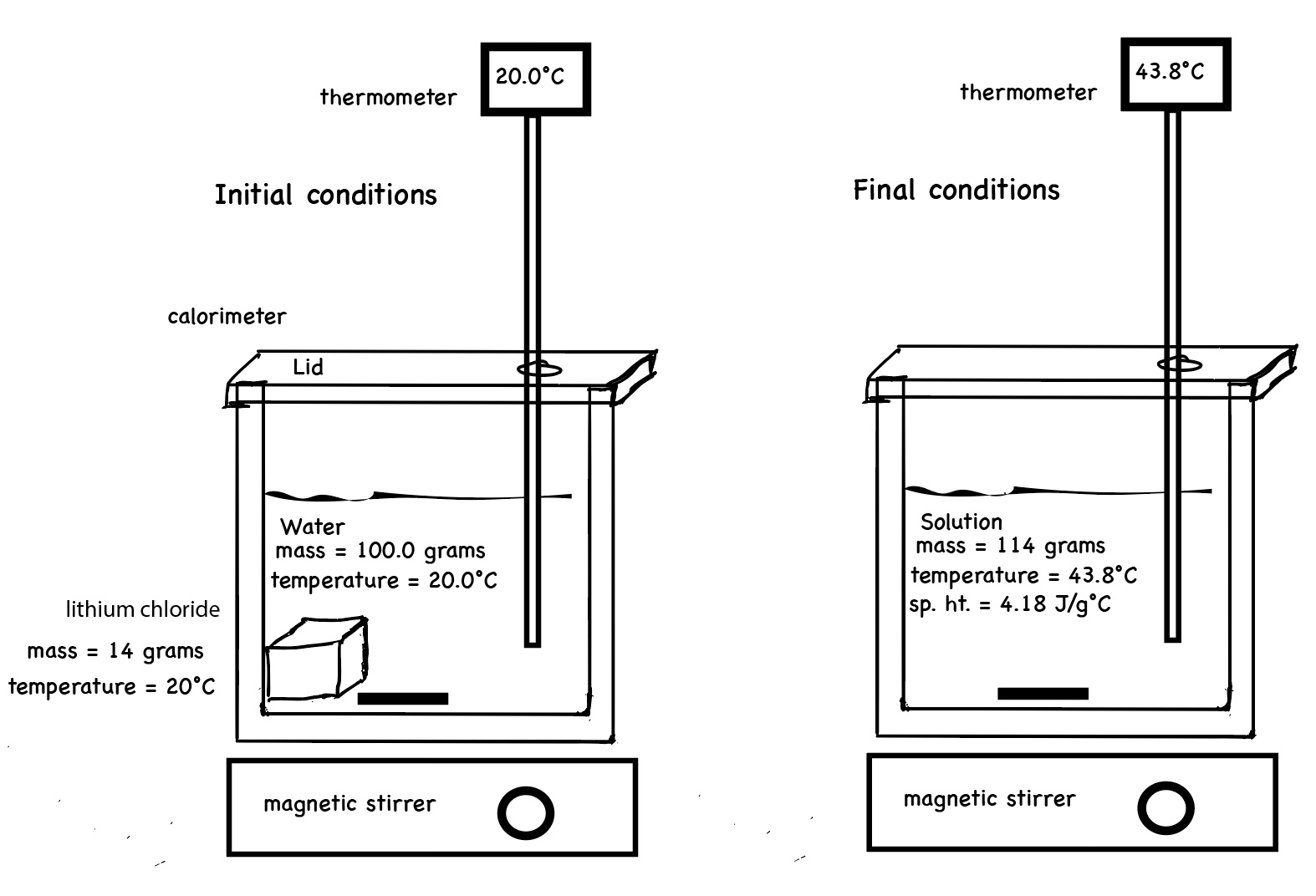
Calorimetry With Two Solutions . Calorimetry is used to measure. the addition of 3.15 g of ba(oh) 2 •8h 2 o to a solution of 1.52 g of nh 4 scn in 100 g of water in a calorimeter. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. For example, when an exothermic reaction. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. this chemistry video tutorial explains how to solve basic calorimetry problems. It discusses how to calculate.
from cider.uoregon.edu
Calorimetry is used to measure. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It discusses how to calculate. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the addition of 3.15 g of ba(oh) 2 •8h 2 o to a solution of 1.52 g of nh 4 scn in 100 g of water in a calorimeter. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. this chemistry video tutorial explains how to solve basic calorimetry problems.
Heat of Solution Calorimetry Simulation Dissolving Salts in Water CIDER
Calorimetry With Two Solutions a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. the addition of 3.15 g of ba(oh) 2 •8h 2 o to a solution of 1.52 g of nh 4 scn in 100 g of water in a calorimeter. this chemistry video tutorial explains how to solve basic calorimetry problems. Calorimetry is used to measure. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It discusses how to calculate.
From www.scribd.com
2. Calorimetry (2) Materials Science Mechanics Calorimetry With Two Solutions one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure. It discusses how to calculate. For example, when an exothermic reaction. one technique we can use to measure the amount of heat involved in a chemical or physical process is known. Calorimetry With Two Solutions.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry With Two Solutions one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure. the addition of 3.15 g of ba(oh) 2. Calorimetry With Two Solutions.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry With Two Solutions Calorimetry is used to measure. For example, when an exothermic reaction. It discusses how to calculate. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. this chemistry video tutorial explains how to solve basic calorimetry problems. a calorimeter is a device used to measure. Calorimetry With Two Solutions.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimetry With Two Solutions For example, when an exothermic reaction. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to. Calorimetry With Two Solutions.
From exoepufyb.blob.core.windows.net
Calorimetry Multiple Choice Questions at Candace Cottingham blog Calorimetry With Two Solutions this chemistry video tutorial explains how to solve basic calorimetry problems. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It discusses how to calculate.. Calorimetry With Two Solutions.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow (M6Q5) UWMadison Calorimetry With Two Solutions this chemistry video tutorial explains how to solve basic calorimetry problems. For example, when an exothermic reaction. the addition of 3.15 g of ba(oh) 2 •8h 2 o to a solution of 1.52 g of nh 4 scn in 100 g of water in a calorimeter. one technique we can use to measure the amount of heat. Calorimetry With Two Solutions.
From saylordotorg.github.io
Calorimetry Calorimetry With Two Solutions the addition of 3.15 g of ba(oh) 2 •8h 2 o to a solution of 1.52 g of nh 4 scn in 100 g of water in a calorimeter. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure. this. Calorimetry With Two Solutions.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimetry With Two Solutions this chemistry video tutorial explains how to solve basic calorimetry problems. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure.. Calorimetry With Two Solutions.
From cider.uoregon.edu
Calorimetry Heat Exchange Hot Metal in Cold Water Real and Computer Simulation CIDER Calorimetry With Two Solutions For example, when an exothermic reaction. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It discusses how to calculate. Calorimetry is used to measure. . Calorimetry With Two Solutions.
From www.scribd.com
calorimetry Calorimetry With Two Solutions one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. the addition of 3.15 g of ba(oh) 2 •8h 2 o to a solution of 1.52 g of nh 4 scn in 100 g of water in a calorimeter. a calorimeter is a device used. Calorimetry With Two Solutions.
From studyadvertiser.z21.web.core.windows.net
How To Use A Calorimeter Stepbystep Calorimetry With Two Solutions the addition of 3.15 g of ba(oh) 2 •8h 2 o to a solution of 1.52 g of nh 4 scn in 100 g of water in a calorimeter. It discusses how to calculate. Calorimetry is used to measure. one technique we can use to measure the amount of heat involved in a chemical or physical process is. Calorimetry With Two Solutions.
From www.scribd.com
Chemsheets As 1047 Calorimetry 2 PDF Heat Capacity Heat Calorimetry With Two Solutions the addition of 3.15 g of ba(oh) 2 •8h 2 o to a solution of 1.52 g of nh 4 scn in 100 g of water in a calorimeter. For example, when an exothermic reaction. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. . Calorimetry With Two Solutions.
From www.chegg.com
Solved You mix two solutions in a coffee cup calorimeter Calorimetry With Two Solutions one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. the addition of 3.15 g of ba(oh) 2 •8h 2. Calorimetry With Two Solutions.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Calorimetry With Two Solutions one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. one technique we can use to measure the amount of heat involved in a chemical or. Calorimetry With Two Solutions.
From psu.pb.unizin.org
Calorimetry (9.2) Chemistry 110 Calorimetry With Two Solutions one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It discusses how to calculate. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a calorimeter is a device used to measure. Calorimetry With Two Solutions.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry With Two Solutions the addition of 3.15 g of ba(oh) 2 •8h 2 o to a solution of 1.52 g of nh 4 scn in 100 g of water in a calorimeter. It discusses how to calculate. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. a. Calorimetry With Two Solutions.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimetry With Two Solutions a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. For example, when an exothermic reaction. one technique we can use to measure the amount of. Calorimetry With Two Solutions.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Calorimetry With Two Solutions For example, when an exothermic reaction. the addition of 3.15 g of ba(oh) 2 •8h 2 o to a solution of 1.52 g of nh 4 scn in 100 g of water in a calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is. Calorimetry With Two Solutions.